Telehealth
Payment policy
COVID-19 Temporary payment policy (includes telehealth)
COVID-19 testing
Fee schedules
Log in and to go Office Resources>Billing & Reimbursement>Fee schedules.
Contact us
(Medical and Mental Health) Network Management and Credentialing Services
1-800-316-BLUE (2583)
Dental Network Management
1-800-882-1178
Links & resources
- Blue Cross Coronavirus Resource Center (public)
- Member coronavirus help line
1-888-372-1970 - Health news from Blue Cross
We are here to support you as you care for your patients—our members. We are in the process of determining what the end of the Massachusetts public health emergency means to our business and our provider partners. We will share additional information when available.
Several changes regarding telehealth and COVID-related care and treatment made during State of Emergency became permanent on January 1, 2021 with the passage of the Patients First Act. We will continue to monitor and assess potential impacts to our business and our provider partners as the state considers any further actions on measures established during the state of emergency.
We have shared the following July 1, 2021 changes with our providers:
- The temporary cost share waiver for non-COVID medical and behavioral telehealth service will expire, reinstating member cost.
- Authorization requirements will resume for Commercial, Federal Employee Program (FEP) and Medicare Advantage plans.
News & updates
Vaccines and treatments
Vaccine billing
Blue Cross Blue Shield of Massachusetts follows federal and state-mandated requirements for COVID-19 treatment coverage.
For our commercial products (managed care HMO and POS, PPO, and Indemnity), we will accept the following CPT codes for COVID vaccines and COVID vaccine administration. For Medicare Advantage plans, you must submit claims for COVID-19 vaccine and the administration of the vaccine to the CMS Medicare Administrative Contractor (MAC) for payment.
Please submit the vaccine administration procedure code and vaccine/toxoid code on the same claim. Since the vaccine is supplied free, we will not reimburse separately for the vaccine, regardless of the modifier.
If a vaccine administration service is provided with an evaluation and management service that:
- Is appended with modifier 25 and is unrelated to the vaccine administration service, Blue Cross will reimburse both services.
- Is not appended with modifier 25 or appended with modifier 25 and is related to vaccine administration service, Blue Cross will deny the evaluation and management service.
This applies to professional and facility claims.
Vaccine and vaccine administration codes for COVID-19
| Code | Service description | Comments |
| 91300 | Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 30 mcg/0.3mL dosage, diluent reconstituted, for intramuscular use | Effective 12/11/2020 (Pfizer-BioNtech COVID-19 vaccine) Since the vaccine is supplied free, Blue Cross will not reimburse separately for the vaccine, regardless of the modifier. |
| 0001A | Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 30 mcg/0.3mL dosage, diluent reconstituted; first dose | Effective 12/11/2020 Bill for administration of first dose of CPT 91300 (Pfizer-BioNtech COVID-19 vaccine) |
| 0002A | Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 30 mcg/0.3mL dosage, diluent reconstituted; second dose | Effective 12/11/2020 Bill for administration of second dose of CPT 91300 (Pfizer-BioNTech COVID-19 vaccine) |
| 91301 | Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 100 mcg/0.5mL dosage, for intramuscular use | Effective 12/18/2020 (Moderna-COVID-19 vaccine) Since the vaccine is supplied free, Blue Cross will not reimburse separately for the vaccine, regardless of the modifier. |
| 0011A | Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 100 mcg/0.5mL dosage; first dose | Effective 12/18/2020 Bill for administration of first dose of CPT 91301 (Moderna-COVID-19 vaccine) |
| 0012A | Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 100 mcg/0.5mL dosage; second dose | Effective 12/18/2020 Bill for administration of second dose of CPT 91301 (Moderna-COVID-19 vaccine) |
| 91303 | Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, DNA, spike protein, adenovirus type 26 (Ad26) vector, preservative free, 5x1010 viral particles/0.5mL dosage, for intramuscular use | Effective 2/27/2021 (Janssen COVID-19 Vaccine) Since the vaccine is supplied free, Blue Cross will not reimburse separately for the vaccine, regardless of the modifier. |
| 0031A | Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, DNA, spike protein, adenovirus type 26 (Ad26) vector, preservative free, 5x1010 viral particles/0.5mL dosage, single dose | Effective 2/27/2021 Bill for administration of CPT 91303 (Janssen COVID-19 Vaccine) |
| 91302 | Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, DNA, spike protein, chimpanzee adenovirus Oxford 1 (ChAdOx1) vector, preservative free, 5x1010 viral particles/0.5mL dosage, for intramuscular use |
Once a COVID-19 vaccine has EUA or approval from the FDA, Blue Cross will accept this vaccine CPT code and administrative codes. (91302 AstraZeneca-COVID-19 vaccine) Since the vaccine is supplied free, we will not reimburse separately for the vaccine, regardless of the modifier. |
| 0021A | Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, DNA, spike protein, chimpanzee adenovirus Oxford 1 (ChAdOx1) vector, preservative free, 5x1010 viral particles/0.5mL dosage; first dose | |
| 0022A | Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, DNA, spike protein, chimpanzee adenovirus Oxford 1 (ChAdOx1) vector, preservative free, 5x1010 viral particles/0.5mL dosage; second dose |
FDA-approved pharmaceutical treatment
Blue Cross Blue Shield of Massachusetts covers all FDA-approved drugs for COVID-19 with no cost share to our members throughout the duration of the public health emergency.
For our Medicare Advantage members, coverage is through original Medicare.
For our commercial products (managed care HMO and POS, PPO, and Indemnity), we will accept the following CPT codes for treatment for COVID-19 infection. For Medicare Advantage plans, you must submit claims for COVID-19 drug and the administration of the drug to the CMS Medicare Administrative Contractor (MAC) for payment.
We are following guidelines from the Blue Cross Blue Shield Association regarding coverage for Federal Employee Program members. For more details please visit fepblue.org.
Since the drug is supplied free, we will not reimburse separately for the drug regardless of modifier.
| Code | Service description | Comments |
| M0245 | Intravenous infusion, bamlanivimab and etesevimab, includes infusion and post administration monitoring | Effective 2/9/2021 |
| Q0239 | Injection, bamlanivimab-xxxx, 700 mg | Effective 11/9/2020 Drug code not reimbursed regardless of modifier |
| Q0243 | Injection, casirivimab and imdevimab, 2400 mg | Effective 11/21/2020 Drug code not reimbursed regardless of modifier |
| Q0245 | Injection, bamlanivimab and etesevimab, 2100 mg | Effective 2/9/2021 Drug code not reimbursed regardless of modifier |
Investigational drugs
Covered investigational drugs
Several drugs are under investigation as potential treatments for COVID-19 that have shown early benefit in clinical trials. Blue Cross Blue Shield of Massachusetts covers the following drugs when used outside a clinical trial for patients who are in an inpatient hospital setting and require treatment beyond respiratory support, at the discretion of their treating provider:
- Antiviral therapy (such as hydroxychloroquine; the original hydroxychloroquine policy still applies – see below)
- Immunomodulators (such as dexamethasone)
Please note that standard inpatient payment policy rules apply.
Chloroquine and hydroxychloroquine (Plaquenil)
On June 5, 2020, the FDA revoked the Emergency Use Authorization (EUA) for hydroxychloroquine and chloroquine for the treatment of COVID-19 because the benefits of using them outweigh the known and potential risks for authorized use. For more information, read these FAQs: Frequently Asked Questions on the Revocation of the Emergency Use Authorization for Hydroxychloroquine Sulfate and Chloroquine Phosphate (PDF, 125 KB)
As of April 1, for the duration of the COVID-19 public health emergency, we have added a 10-day supply limit to these medications for:
- Members who are newly prescribed the medication for rheumatological and dermatological use (for example, to treat lupus, malaria, rheumatoid arthritis).
This supply limit applies to members who use our standard Blue Cross Blue Shield of Massachusetts formulary.
Quantity
You can request an authorization to cover more. To make this request, please submit the Massachusetts Standard Form for Medication Prior Authorization Requests (click the link and find the form by choosing Authorization – Pharmacy). Or, contact our Clinical Pharmacy Operations area.
Rheumatological and dermatological use
Members who filled a prescription for rheumatological and dermatological use within the previous 180 days are excluded from the quantity limit. We’re contacting them to suggest that they take advantage of our early refill policy during this public health emergency, so they can have a supply of their medication. Some members may also contact you for a prescription for up to a 90-day supply from the Express Scripts Pharmacy®' (mail order).
Why we made the change
You may be aware that on March 30, 2020, the Food and Drug Administration (FDA) issued an emergency authorization to use chloroquine and hydroxychloroquine as experimental coronavirus treatment.
The Massachusetts Division of Insurance (DOI) issued a March 26, 2020 Bulletin addressing this topic. The DOI asked insurers to continue covering these medications for rheumatologic or dermatologic conditions under their current policies. For COVID-19-related diagnoses, they asked insurers to add quantity limits.
Keep in mind
- For Federal Employee Program and Medicare Advantage members, coverage for these drugs remains the same at this time.
- For members using the National Preferred Formulary (managed by Express Scripts, Inc.), there are new quantity limits for these medications.
Non-covered investigational drugs
Blue Cross Blue Shield of Massachusetts does not cover drugs under investigation through clinical trials that have not demonstrated improvement in patient outcomes in early studies or are not recommended for use outside of the clinical trial setting by the Centers for Disease Control and Prevention (CDC), National Institutes of Health (NIH), or Department of Public Health (DPH) guidelines.
The following drugs are not covered outside of the clinical trial setting:
- Blood-derived products (such as SARS-CoV-2 immunoglobulins, mesenchymal stem cells)
- Antiviral therapies or immunomodulators without published supporting evidence (such as lopinavir/ritonavir, other HIV protease inhibitors)
- Other therapies currently under investigation without published supporting evidence
Medication refills
We lifted limits on early refills of most prescription medications, allowing members to obtain one additional fill of their existing prescription. This is at the discretion of the prescriber and/or dispensing pharmacist. At the same time, Blue Cross Blue Shield of Massachusetts continues to monitor and comply with all applicable state and federal regulations, including regulation of opioid prescribing and dispensing.
Cognitive rehabilitation
Per state mandate Chapter 260 of the Acts of 2020 – Patients First Act, cognitive rehabilitation for cognitive impairment resulting from COVID-19 is covered in the outpatient setting.1
Providers should document ALL of the following for coverage:
- Cognitive impairments resulted from COVID-19 that was either clinically diagnosed or diagnosed through PCR/Antigen testing, AND
- Patient symptoms impair daily functioning and are unlikely to resolve on their own over time, AND
- Patient symptoms are expected to improve with cognitive rehabilitation.
Inpatient cognitive rehabilitation for cognitive impairment resulting from COVID-19 is not covered unless the patient otherwise meets criteria for inpatient level of care.
For more information, see Medical Policy 660: Cognitive Rehabilitation
Telehealth (telephone calls and video visits)
Coverage
We cover medically necessary telehealth services (COVID-19 and non-COVID-19-related) for in-network providers. We've remove dmember cost(copayments, co-insurance, and deductibles) for all telehealth services, including behavioral health.
We reimburse providers at the same rate as we reimburse a face-to-face visit, as long as it meets clinical standards, for the duration of the Massachusetts public health emergency.
Please refer to the COVID-19 Temporary payment policy for telehealth billing guidelines.
Federal Employee Program (FEP) members
We removed the member cost for all telehealth services (COVID-19 and non-COVID-19-related) received through the Teladoc network. Members can register for Teladoc by visiting fepblue.org/coronavirus. For providers not in the Teladoc network, the applicable cost share applies (unless COVID-19 related).
Platforms and technologies
All Blue Cross Blue Shield of Massachusetts contracted doctors and health care providers can provide care remotely, using any technology, for medically necessary covered services (COVID-19 AND non-COVID-19 related) to our members. This includes visits by phone and your communication platform of choice. You don’t need to be part of a telehealth network of providers to offer this.
During the Massachusetts public health emergency, we reimburse all providers, including ancillary, behavioral health, and applied behavioral analysis providers, at the same rate they would receive for an in-person visit. This is in place for the duration of the Massachusetts state of emergency.
The U.S. Department of Health and Human Services and the Office of Civil Rights have relaxed HIPAA requirements related to the use of telehealth services during the COVID-19 nationwide public health emergency. See the Notification of Enforcement Discretion for telehealth.
Billing
To bill for telehealth, follow the same telehealth billing guidelines as you would for an in-person visit and include the following modifiers with the applicable place of service as outlined in the COVID-19 Temporary payment policy:
- Practitioners must use modifier GT, 95, G0, or GQ to designate that that they are providing services via synchronous/asynchronous telehealth audio and/or video telecommunications systems rather than an in-person encounter.
- Your claim represents your attestation that you provided the service to the patient via telehealth.
- When reporting modifier GT, 95, G0, or GQ, the practitioner is attesting that services were provided via synchronous/asynchronous telehealth audio and/or video telecommunications systems.
- If you are submitting 1500 claims using Direct Data Entry in Online Services, please do not use separate fields for each character of the modifier. The screenshot below shows the correct way to enter modifiers.
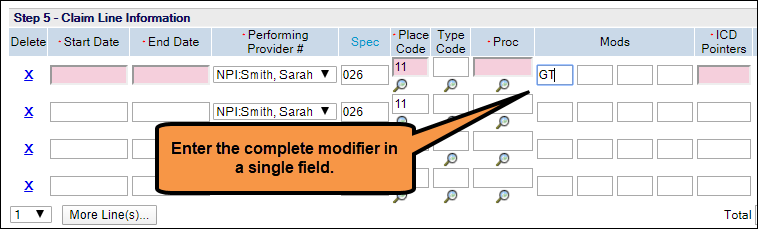
Bill for telephonic services using the additional billing guidelines and applicable place of service codes in our COVID-19 Temporary payment policy.
Ancillary and some behavioral health providers
Important note: This information only applies to the ancillary and behavioral health specialties on this list.
When you provide any services by phone, do not bill the specific telephonic CPT codes. Bill all covered services that you render either by telehealth/video or telephone as if you are performing an in-person service using the codes that are currently on your fee schedule.
You must use one of the following telehealth modifiers listed above (GT, 95, G0, and GQ) and the applicable place of service code. We’ll allow the use of these modifiers for any service on your fee schedule. This will enable us to pay you the same rate we pay you for in-person visits.
Acute care hospitals
When you provide telehealth or telephonic services, bill on a facility claim using a professional revenue code with the telehealth services outlined in our COVID-19 Temporary payment policy. Use one of the following telehealth modifiers on all lines billed: GT, 95, G0, or GQ. Bill as if you are performing an in-person service, using the revenue and HCPCS/CPT code combinations that you would normally bill on a facility claim.
Note: Telephonic codes (98966-98968, 99441-99443) do not require the use of any telehealth modifier.
Medicare Advantage facilities should follow CMS guidelines for telehealth services.UB-04 billing
UB-04 billers do not need to enter a place of service when billing for services provided by phone.
Please see our COVID-19 Temporary payment policy for more information.
Criteria for offering telehealth
You can offer telehealth as long as you are contracted and credentialed by Blue Cross Blue Shield of Massachusetts. There are no additional credentialing or contracting processes you need to follow to offer telehealth services.
The U.S. Department of Health and Human Services and the Office of Civil Rights have relaxed HIPAA requirements related to the use of telehealth services during the COVID-19 nationwide public health emergency. See the Notification of Enforcement Discretion for telehealth.
Patient Age
There are no age limits for members who need care through telehealth or phone services. You can have telehealth video or phone visits with children, adolescents, and adults.
Out-of-state provider billing
Please consult your local Blue plan you are contracted with. They have their own payment policy for telehealth services. Blue Cross Blue Shield of Massachusetts will reimburse telehealth covered claims that we receive through the BlueCard® program.
COVID-19 testing and care
APR-DRG Grouper ICD-10 code updates
Effective for claims with discharge dates or dates of service on or after April 1, 2020, for all commercial products, we have updated our APR-DRG grouper with the ICD-10 diagnosis codes below. This update also includes the ICD-10 vaping-related disorder code.
| Diagnosis code | Description |
|---|---|
| U07.1 | COVID-19 virus identified |
| U07.0 | Vaping-related disorder |
Drive-through/temporary COVID-19 testing
When testing patients in a drive-through or other temporary setting (such as a tent), please use the following codes for claims with dates of service on or after March 1, 2020.These codes apply to all commercial, Medicare Advantage, and Federal Employee Program (FEP) members.
| Code | Comments |
|---|---|
| 99001 (CPT) |
Blue Cross Blue Shield of Massachusetts is temporarily allowing reimbursement for this code for drive-through testing specimen collection* |
| G2023 (HCPCS) |
Specimen collection for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Coronavirus disease [COVID-19]), any specimen source* |
*Reimbursement for these codes is included in the payment for an evaluation or management (E/M) service if reported by the same provider on the same day, for the same member.
Please also use one of the following applicable place of service codes that describes the location of the drive-through or temporary testing site.
| Place of service code | Location |
|---|---|
| POS 11 | Office |
| POS 15 | Mobile unit |
| POS 20 | Urgent care facility |
| POS 22/19 | On/off campus outpatient hospital |
| POS 23 | Emergency room hospital |
Testing coverage
Blue Cross Blue Shield of Massachusetts follows federal and state requirements for SARS CoV-2 (COVID-19) testing coverage.
Commercial members: Managed care (HMO and POS), PPO, and Indemnity
Viral testing
Reverse transcription-polymerase chain reaction (RT-PCR) or antigen testing to detect the presence of SARS-CoV-2 for the diagnosis of COVID-19 is covered when ordered by a health care provider who is making an individualized clinical assessment of the patient in accordance with current standards of medical practice. This includes the Centers for Disease Control (CDC) and the Massachusetts Department of Public Health (DPH) guidelines.
Covered tests must be approved by the FDA or have Emergency Use Authorization, or the developer must have requested, or intends to request Emergency Use Authorization approval.
In accordance with the Centers for Disease Control (CDC) and the MA Department of Public Health guidelines, covered scenarios include (but are not limited to):
- Symptoms consistent with COVID-19, such as fever, cough, shortness of breath, chills, muscle pain, sore throat, anosmia, and gastrointestinal distress
- Asymptomatic patients with direct exposure and/or close contact to another individual with a confirmed case of COVID-19
- Close contact is defined by the CDC as someone who was within 6 feet of an infected person for a cumulative total of 15 minutes or more over a 24-hour period* starting from 2 days before illness onset (or, for asymptomatic patients, 2 days prior to test specimen collection) until the time the patient is isolated
- Asymptomatic patients who have been identified by contact tracing
- Symptomatic or asymptomatic patients who require testing prior to a medical procedure or surgery
- Admission to a facility – including but not limited to a hospital operated or licensed by the Department of Public Health or Mental Health, a long-term acute care hospital, or a skilled nursing facility
* Individual exposures added together over a 24-hour period (e.g., three 5-minute exposures for a total of 15 minutes).
Covered testing sites include (but are not limited to):
- Drive-through testing sites
- Emergency rooms
- Medical provider offices
- The patient’s home (using a testing kit—a patient self-swab)
What’s not covered
PCR or antigen testing to detect SARS-CoV-2 is not covered in the following scenarios:
- General screening purposes such as
- return-to-work
- to attend school, day care, or camp
- For public health or surveillance purposes
- For periodic or serial testing of asymptomatic high-risk individuals (examples include congregate housing and occupational safety)
- Tests that have been denied FDA approval, an Emergency Use Authorization from the FDA, or laboratories that have not submitted an Emergency Use Authorization request within a reasonable timeframe
- Member transportation to or from testing sites (unless the member meets requirements for ambulance services)
Antibody testing
Serologic testing for the presence of SARS-CoV-2 IgM/IgG antibodies is covered for FDA and Emergency Use Authorization tests (as described above) when ordered by a health care provider who is making an individualized clinical assessment of the patient in accordance with current standards of medical practice, including the Centers for Disease Control (CDC) and Massachusetts Department of Public Health (DPH) guidelines.
According to the CDC, serologic testing:
- Should not be used to determine immune status in individuals
- Should not be used to determine if someone can return to work.
- Should not be used to group people together in settings such as schools, dormitories, and correctional facilities.
- Can be offered as a method to support a diagnosis of acute COVID-19 illness for persons who present late. For persons who present 9-14 days after the onset of illness, serologic testing can be offered in addition to recommended direct detection methods, such as polymerase chain reaction.*
- Should be offered as a method to help establish a diagnosis when patients present with late complications of COVID-19 illness, such as multisystem inflammatory syndrome in children
* Detection of specific antibody in serum, plasma, or whole blood that indicates new or recent infection provides presumptive laboratory evidence of COVID-19 illness, according to the Council of State and Territorial Epidemiologists (CSTE) interim case definition for COVID-19.
Serologic testing for the presence of antibodies is not covered
- For general screening purposes such as:
- return-to-work
- to attend school, day care, or camp
- for public health or surveillance purposes
- for periodic or serial testing of asymptomatic individuals (examples include congregate housing such as dormitories and residential facilities, and occupational safety)
- For tests that have been denied FDA approval, an Emergency Use Authorization from the FDA, or laboratories that have not submitted an Emergency Use Authorization request within a reasonable timeframe
- For member transportation to or from testing sites (unless the member meets requirements for ambulance services)
- To screen for eligibility to donate plasma
Medicare HMO BlueSM and Medicare PPO BlueSM Members
In keeping with CMS guidance issued September 2, 2020 and for the duration of the COVID-19 public health emergency, Blue Cross will cover, without a healthcare professional’s order, the cost of one diagnostic test for COVID-19 and one diagnostic test each for influenza virus or similar respiratory condition for Medicare members when performed in conjunction with a COVID-19 test and needed to obtain a final COVID-19 diagnosis. Subsequent tests will require the order of an authorized health care professional.
Viral testing
The use of reverse transcription-polymerase chain reaction (RT-PCR) or antigen testing to detect the presence of SARS-CoV-2 for a diagnosis of COVID-19 infection is covered for FDA-approved tests when ordered by any healthcare professional authorized under state law.
Antibody testing
Serologic testing for the presence of antibodies for known or suspected current or prior COVID-19 infection is covered for FDA-approved tests when ordered by any healthcare professional authorized under state law.
Federal Employee Program
The Federal Employee Program (FEP) covers COVID-19 testing and antibody testing with no member cost share, regardless of provider status, including testing for:
- travel purposes
- asymptomatic members
- pre-surgical testing for elective and non-elective procedures
- return-to-work
Note: There are no limits on frequency of testing.
Testing codes
Below are the codes for providers and laboratories to test patients for COVID-19. They apply to commercial, Federal Employee Program, and Medicare Advantage members.
| Code | Description | Reimbursement date |
|---|---|---|
| U0001 (HCPCS) |
CDC 2019 novel coronavirus (2019-ncov) real-time rt-pcr diagnostic panel | Effective April 1, 2020 for dates of service on or after February 4, 2020 |
| U0002 (HCPCS) |
2019-nCoV Coronavirus, SARS-CoV-2/2019-nCoV (COVID-19), any technique, multiple types or subtypes (includes all targets), non-CDC | |
| U0003 (HCPCS) |
Infectious agent detection by nucleic acid (DNA or RNA); severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Coronavirus disease [COVID-19]), amplified probe technique, making use of high throughput technologies as described by CMS-2020-01-R. | Reimbursable for dates of service on or after April 14, 2020
Only to be reported with use of high-throughput technologies. See our COVID-19 Temporary payment policy |
| U0004 (HCPCS) |
2019-nCoV Coronavirus, SARS-CoV-2/2019-nCoV (COVID-19), any technique, multiple types or subtypes (includes all targets), non-CDC, making use of high throughput technologies as described by CMS-2020-01-R. | |
| 87635 (CPT) |
Infectious agent detection by nucleic acid (DNA or RNA); severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Coronavirus disease [COVID-19]), amplified probe technique | Effective March 13, 2020
Do not bill 87635 and U0002 on the same day for the same patient |
We have added these codes to our COVID-19 Temporary payment policy.
Diagnosis codes
Use the diagnosis codes below for patients presenting for evaluation of suspected
We expect providers to code for COVID-19 testing and treatment using guidelines provided by the CDC. Blue Cross will identify patients presenting for evaluation of possible COVID-19 using the below codes*:
| If patient is | Please use | Definition |
|---|---|---|
| Asymptomatic and without known COVID-19 contact | Z20.828 | Contact with and (suspected) exposure to other viral communicable diseases |
| Symptomatic or has been exposed to COVID-19 | Z03.818 | Encounter for observation for suspected exposure to other biological agents ruled out |
| Z11.59 | Contact with and (suspected) exposure to other viral communicable diseases | |
| Z11.52 | Encounter for screening for COVID-19 (Effective January 1, 2021) | |
| Z20.822 | Contact with and (suspected) exposure to COVID-19 (Effective January 1, 2021) |
If your patient has previously confirmed COVID-19 illness or tests positive for COVID-19, use the code below.
| Diagnosis code | Service description | |
|---|---|---|
| B97.29 | Other coronavirus as the cause of diseases classified elsewhere | |
| B97.21 | SARS-associated coronavirus as the cause of diseases classified elsewhere | |
| U07.1 | 2019-nCOV acute respiratory disease (Effective April 1, 2020) | |
| B342 | Coronavirus infection, unspecified | |
| J12.82 | Pneumonia due to COVID-19 (Effective January 1, 2021) | |
| M35.81 | Multisystem inflammatory syndrome (Effective January 1, 2021) | |
| M35.89 | Other specified systemic involvement of connective tissue (Effective January 1, 2021) | |
*The CDC has created an interim set of ICD-10 CM official coding guidelines, effective February 20, 2020.
Referrals and authorizations
Extended authorizations for deferred services
Expiration of extended authorizations for deferred services on 12/31/20
In 2020, we extended time-limited authorizations through the end of the year for specific outpatient procedures that our members may not have been able to receive due to the COVID-19 emergency. New authorizations will be required for services deferred into 2021, and all other administrative requirements related to these services continue to apply.
Assisted reproductive technology services
For assisted reproductive technology services listed in our medical policy that require prior authorization:
- We extended existing authorizations issued for the first six months of 2020 to December 31, 2020. If you plan to provide a previously approved service to a patient in 2021, please call our Clinical Intake Department at the appropriate number and we will create a new authorization or update the existing one.
- We now give 180 days for services to be completed on new authorizations. After that time, an authorization extension is required.
Durable medical equipment
We extended existing authorizations through December 31, 2020. If you plan to provide a previously approved service to a patient in 2021, please call our Clinical Intake Department at the appropriate number and we will create a new authorization or update the existing one.
High-technology radiology and obstructive sleep apnea testing and treatment
For the high-technology radiology and sleep testing and treatment services that require prior authorization with AIM Specialty Health, during the public health emergency, we authorized new requests for 180 days to allow time to have services performed. Effective January 1, 2021, AIM will return to standard processes and authorize services for 60 days.
Neuropsychological testing services
For neuropsychological testing services listed in our medical policy that require prior authorization, we typically give the member 365 days to complete the authorized services. However, we’ve extended existing authorizations for the period of March 1, 2019 – December 31, 2019 to December 31, 2020. If you plan to provide a previously approved service under an authorization that expired on December 31, 2020 to a patient in 2021, please call our Clinical Intake Department at the appropriate number and we will create a new authorization or update the existing one.
Non-emergency ground ambulance transport
We have waived pre-authorization requirements for ground ambulance transport by a contracted provider. In addition, ground ambulance transport to and from the locations listed below is covered to help our healthcare delivery system optimize inpatient capacity.
- Applies to in-network, ground ambulance providers for HMO, PPO, Indemnity, Medicare Advantage, and Federal Employee Program* members
- Excludes air ambulance transport
- Notification is not required
- Cost share is waived for members with a COVID-19 diagnosis
- Cost share will apply to members without a COVID-19 diagnosis
Bill for ambulance transport
Be sure to bill using CPT A0426, A0428, A0433, or A0434 (non-emergent transports) and the appropriate modifier shown below to represent the direction of the transfer.
| Modifier | Description |
|---|---|
| DH | Diagnostic site (including COVID-19 testing) or therapeutic site (including dialysis; excluding physician office or hospital) to hospital |
| EH | Residential, domiciliary, custodial facility (other than skilled nursing facility) if the facility is the beneficiary’s home to hospital |
| HD | Hospital to diagnostic site (including COVID-19 testing) or therapeutic site (including dialysis; excluding physician office or hospital) |
| HE | Hospital to residential, domiciliary, custodial facility (other than skilled nursing facility) if the facility is the beneficiary’s home |
| HH | Hospital to hospital (includes ASCs approved to provide hospital level of care) |
| HN | Hospital to alternative site for skilled nursing facility (SNF) |
| HR | Hospital to residence |
| JN* | Freestanding end-stage renal disease (ESRD) facility to skilled nursing facility |
| NH | Alternative site for SNF to hospital |
| NJ* | Skilled nursing facility to freestanding end-stage renal disease (ESRD) facility |
| NN | SNF to SNF |
| NR* | SNF to residence |
| PD | Physician office to community mental health center, federally qualified health center, rural health center, urgent care facility, non-provider-based ambulatory surgical center or freestanding emergency center, or location furnishing dialysis services that is not affiliated with an end-stage renal facility |
| PE* | Physician office to residential, domiciliary, custodial facility (other than skilled nursing) if the facility is the beneficiary’s home |
| PH | Physician office to hospital |
| PR* | Physician office to home |
| RH | Residence to hospital |
| RN* | Residence to SNF |
*These modifiers do not apply to Federal Employee Program members.
COVID-19 testing and treatment
Referrals and prior authorizations are not required for medically necessary testing and treatment for COVID-19.
Blue Cross also removes all referral and authorization requirements for outpatient care if a member is being evaluated or treated for suspected or confirmed COVID-19. This applies to in- and out-of-network providers and to in-person and telehealth/virtual/visits by phone.
Inpatient services
In alignment with guidance from the Division of Insurance, we have resumed the normal authorization processes for all services for our commercial and Federal Employee Program members, and will start requiring authorization for Medicare Advantage members on July 1, 2021.
We will continue to waive the authorization requirement for commercial and Medicare Advantage initial requests for the following services with a COVID diagnosis:
- Emergent inpatient services
- Skilled nursing, rehab, and long-term acute care
- Home health care
If you aren’t already, please submit clinical information for all authorization requests with the exceptions noted above. The authorization process will officially resume for all products effective July 1, 2021.
Administrative changes & other updates
Claims for laboratory services including COVID-19 testing
On or after July 1, 2021, the ordering clinician NPI will be a required field on your claim to indicate that the lab test is medically necessary. This change is described in our April 30, 2021 News Alert, "Lab claims must include ordering clinician NPI starting July 1."
Adjustments to Medicare Advantage reimbursement
As part of the Coronavirus Aid, Relief, and Economic Security (CARES) Act, the Centers for Medicare & Medicaid Services (CMS) has extended the suspension of the mandatory payment reductions known as “sequestration” through December 31, 2021. Beginning on January 1, 2022, sequestration will be reinstituted.
Time to file initial claim submissions
We extended the filing limit for initial claim submissions.
For dates of service between March 1, 2020 and May 31, 2020, you had 150 days from the date of service or the date of discharge (for inpatient stays) to submit your claims for HMO/POS, Medicare Advantage, and PPO members.
We resumed our usual 90-day timely filing limit for dates of service or dates of discharge on and after June 1, 2020.
There is no change to the timely filing guidelines for Indemnity claims.
This policy update applies to all medical providers.
Expedited credentialing and enrollment
We are making every effort to credential providers within seventy-two (72) hours of the date we receive your application.
Simply fill out our Public Health Emergency Credentialing Application (PHE App). Then have an authorized representative of the group you are joining sign the form and send it back to PHEexpeditedCred@BCBSMA.com.
Providers who are approved under this process will receive a Welcome Letter with their effective date.
Member costs (copays)
Telehealth
Effective July 1, 2021, we reinstated member cost copayments, co-insurance, and deductibles for non-COVID telehealth visits, including all mental and behavioral health services. Member cost share continues to be waived for COVID-19 related telehealth visits provided by in-network providers.
Note: These changes do not apply to our Medicare Advantage members. We are following guidelines from the Blue Cross Blue Shield Association regarding coverage for Federal Employee Program members. For more details, please see fepblue.org.
Please bill members for their cost share once the claim has processed
When you are checking eligibility, Online Services will show the standard telehealth cost share. The system will not distinguish between a COVID visit and a non-COVID visit; therefore, we recommend that you bill the member for the applicable cost share once the claim has processed to ensure you do not have to reimburse the member.
Federal Employee Program
For Federal Employee Program members, we've removed the member cost for all telehealth services (COVID-19 and non-COVID-19-related) received through the Teladoc network. Members can register for Teladoc by visiting fepblue.org/coronavirus. For providers not in the Teladoc network, the applicable cost share will apply (unless COVID-19 related).
Medicare Advantage members
Effective January 1, 2021 Medicare Advantage members will have coverage for telehealth services for PCP, specialist, urgent care, and outpatient mental health services. Member cost will be the same as an in-person office visit, and cost will not be waived for a COVID-19 diagnosis.
COVID-19 testing, counseling, vaccination & treatment
We removed all member cost for in-person doctor, urgent care, and emergency room visits related to the testing, counseling, vaccination, and treatment of COVID-19.
For Federal Employee Program (FEP) members, member cost is removed for inpatient acute care hospitals, inpatient rehab facilities, long-term acute care hospitals, and skilled nursing facilities for services related to COVID-19.
FEP will determine coverage for the vaccine once it becomes available.
Inpatient care for COVID-19 diagnoses
Retroactive to March 6, 2020, we waive member cost (copayments, deductible, co-insurance) for medically necessary inpatient acute care hospital services when the claim includes a diagnosis of COVID-19. This will apply to in- and out-of-network services received at an acute care hospital.
It does not include care received at chronic care and long-term acute care hospitals, psychiatric facilities, rehabilitation hospitals, skilled nursing facilities, and substance use disorder facilities.
This policy applies to Blue Cross Blue Shield of Massachusetts members* in the following plans:
- Commercial HMO/POS and PPO (fully insured accounts)
- Federal Employee Program
- Indemnity
- Medex
- Medicare Advantage
Note: Employers who are self-insured may choose not to offer waived cost share for their employees. When the claim processes and you receive your Provider Detail Advisory, you’ll know whether the member has a cost to collect.
*Blue Plan members receiving care in Massachusetts are covered according to their Home plan’s benefits and coverage.
New covered services
- Coronavirus Resource Center at bluecrossma.com/coronavirus.
- FEP members: fepblue.org/coronavirus
Member questions
Members can call our dedicated coronavirus help line at 1-888-372-1970.
Mental health
Copayments
We have removed member cost (copayments, co-insurance, and deductibles) for medically necessary telehealth (virtual video/audio) services or visits by phone for behavioral health services. The COVID-19 Temporary payment policy applies.
We will reimburse medically necessary telehealth and visits by phone at the same rate as an in-person visit, for all providers, including behavioral health providers.
Member cost still applies for an in-person, outpatient visits and for inpatient and residential services.
Billing for partial hospitalization and intensive outpatient using telehealth
You can bill all services for which you are contracted using the telehealth codes with the telehealth modifier.
How to bill for telehealth and services by phone
Blue Cross covers mental health visits by telehealth (video/virtual) or by telephone (“telephonic visits”) throughout the Massachusetts public health state of emergency. Please follow the billing instructions outlined in our COVID-19 Temporary payment policy.
To bill for telehealth/video services during the state of emergency
- Bill the same as you would for in-person visits, and include the following modifiers with the applicable place of service code*: modifier GT, 95, G0, or GQ via synchronous/asynchronous telehealth audio and/or video telecommunications systems to differentiate a telehealth (telemedicine) encounter from an in-person encounter with the patient.
- When reporting modifier GT, 95, G0, or GQ, you are attesting that services were rendered to a patient via synchronous/asynchronous telehealth audio and/or video telecommunications systems.
When reporting the telehealth modifier, if applicable, please place the telehealth modifier after the license modifier.
*UB-04 billers do not need to submit a place of service code.
To bill for services by phone
How you will bill for services by phone depends upon your specialty. See below for details.
If you are this provider type |
Follow these billing instructions |
|---|---|
|
Do not bill the specific telephonic CPT codes.
This will enable us to pay you the same rate we pay you for in-person visits. *UB-04 billers do not need to submit place of service code. |
|
Use the telephonic CPT codes as indicated in the telehealth billing guidelines with the applicable place of service code*.
The billing guidelines are included in the COVID-19 Temporary payment policy *UB-04 billers do not need to submit a place of service code. |
Telehealth claims errors
If you were not reimbursed correctly, or your advisory shows that a member is responsible for a copayment, you can either:
- Wait for our systems to identify the claim and correct it
- Call Provider Service and ask us to reprocess the claim at one of the following toll-free numbers
- 1-800-882-2060 (Physicians)
- 1-800-451-8123 (Hospitals)
- 1-800-451-8124 (Ancillary providers)
Licensure changes
At this time, there are no changes to our licensure requirements.
UB-04 billers: place of service
UB-04 billers do not need to enter place of service codes when billing for telephonic services.
This applies to all accounts except the Federal Employee Program (FEP).
Federal Employee Program patients
Effective March 10, 2020, we expanded the telehealth benefit and removed the member cost (copayments, co-insurance, and deductibles) for all COVID-19 related telehealth services, member cost will apply when billed with the appropriate modifiers.
Find additional coding information on Provider Central.
Member costs are being waived for all Teladoc visits (COVID-19 and non-COVID-19) during this emergency period.
To learn more about Teladoc, visit https://www.teladoc.com/providers/
Dental
Billing limitations for CDT 0140
We do not have any restrictions on the video or voice platform the dentist can use.
Member eligibility
You can use Dental Connect for Providers to verify member eligibility and benefits. If you haven’t used Dental Connect before, you’ll need to register for Dental Connect using partner code BCMA01DPS (this is an important step for registration; Blue Cross Blue Shield of Massachusetts sponsors monthly fees for this service. Or, you can call Dental Provider Services at 1-800-882-1178.
Dental telehealth cost share
Members who already have coverage for problem-focused exams (D0140) will have no cost share (deductible, copayment, or co-insurance).*
*For the Federal Employee Program, benefits and cost share are applicable according to the member’s plan.


